This course is designed to the fundamental concepts and general principles applied to the understanding of the behavior of matter. The course will introduce the theoretical and experimental approaches to the study of the physical properties of chemical systems and their reactions at the macroscopic and atomic/molecular levels. The course will also develop problem solving skills of students in the major areas of Physical Chemistry. In particular, this course provides the foundations in chemical thermodynamics, physical and chemical equilibria, and an introduction to statistical thermodynamics.
The ultimate objective of this course is to understand the structure, properties and transformations of matter, from macroscopic behavior to mechanisms that operate at molecular level. Students in this course will understand the role of this course in relation to other fields. This course will also provide scientific basis for the students to understand the process of collecting, selecting and analyzing experimental data from all areas of chemistry and use this information to construct models capable of predicting new phenomena. Based on concepts from chemistry, physics and mathematics, physical chemistry contributes to many areas of science as diverse as medicine, molecular biology, molecular engineering, chemical engineering, materials and earth sciences.
At the end of the course, the students must be able to:
a. Apply the models for ideal and real gases to chemical problems.
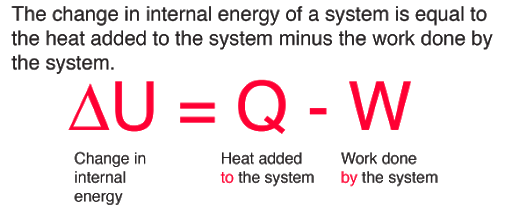
b. Apply the laws of thermodynamics to physical and chemical processes.
c. Construct and interpret phase diagrams for substances and mixtures.
d. Understand the derivation of equilibrium properties of macroscopic
systems from microscopic properties.
- Teacher: Deomila Basnig
- Teacher: Rey Capangpangan
